An efficient path to CE marking
Convenient monitoring of urological health parameters at home – that is the mission of the start-up Streamcheck. Harro Höfliger’s Device Services supported the path to market approval of their smart medical device.
Highly informative data for early detection
A conversation among friends on vacation. Prof. Dr. med. Stefan Siemer reports on his daily work at the Clinic and Polyclinic for Urology and Pediatric Urology at Saarland University and concludes: “If our male patients didn’t wait to see a doctor until they have complaints and made better use of preventive care, we could start therapy early and prevent many an operation. But very often urological check-ups are a taboo subject.” This message resonates with entrepreneur Sebastian Heidrich. His idea is to devise a user-friendly technical solution that helps people to regularly monitor important health data from the comfort of their own home and seek medical advice if necessary.
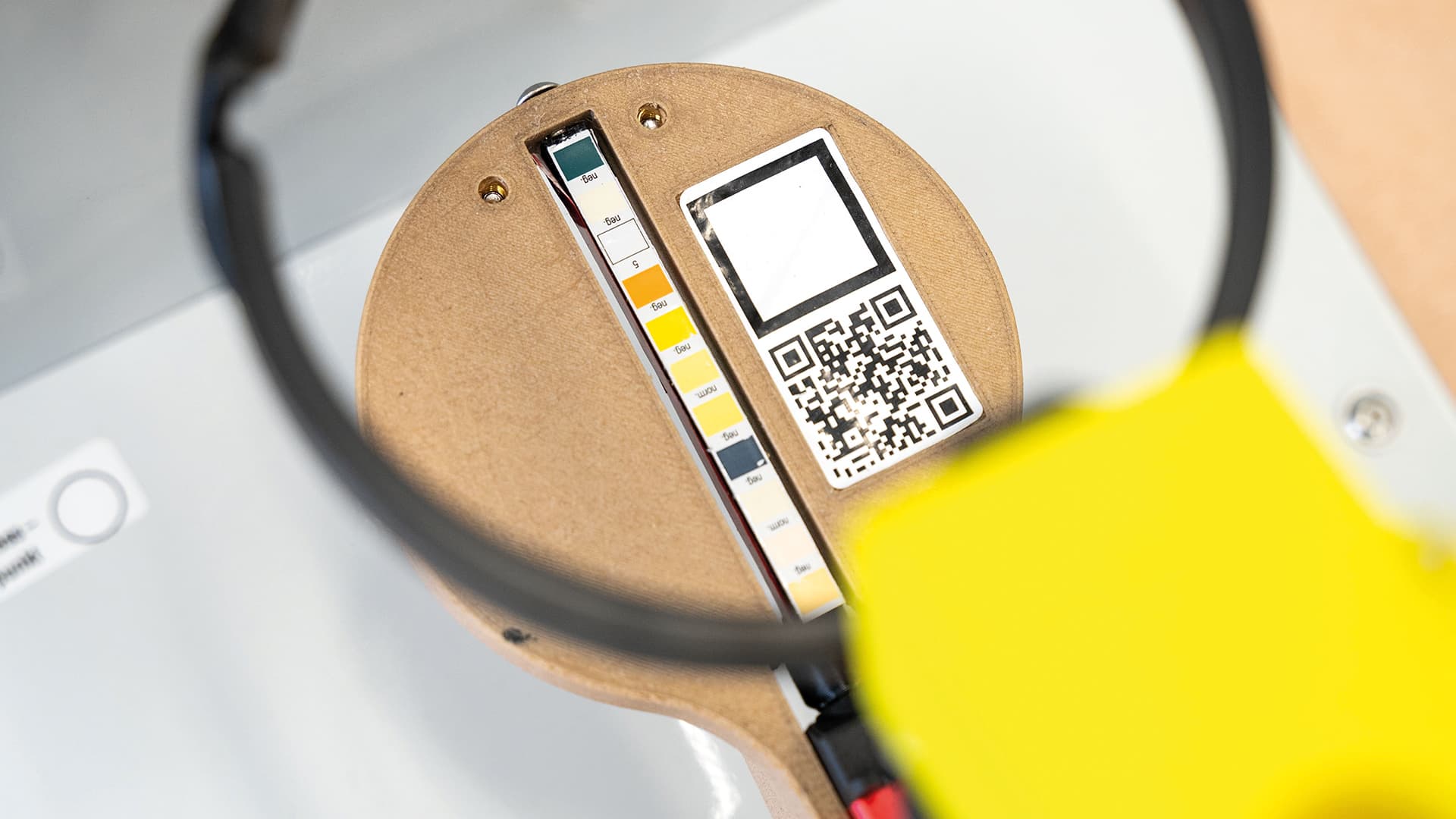
Siemer and Heidrich, together with marketing specialist Sebastian Janus, founded Streamcheck GmbH as a start-up in Vierkirchen, Saxony in 2023. The name says it all: The diagnostic product, developed by an interdisciplinary and steadily growing team, consists of the device and a special cup, and measures the flow rate and volume of urination. An integrated test strip reveals important biomarkers in the urine – such as protein, glucose and nitrite. All data is recorded, analyzed and stored in a smartphone app.
“If urine flows noticeably slowly, this may indicate a condition affecting the prostate, urethra, or bladder,” says Prof. Dr. med. Stefan Siemer. “As a diagnostic procedure, uroflowmetry is established in all urological practices. However, our approach is preventive: If urinary flow is measured regularly at short intervals, let’s say once a month, the first signs of health changes will not go unnoticed. The same applies to biomarkers. If, for example, the device detects white blood cells in the urine, this may indicate inflammation. In either case, it’s a signal for users to have any irregularities checked by a doctor.”
The challenge of CE marking
The innovative Streamcheck system has also attracted the interest of specialist practices and clinics. Harro Höfliger came into play to accelerate the process of market approval as a medical device in the European Union. “We moved forward purposefully and energetically, fine-tuning the technical details. But all the regulations, standards, guidelines and directives to be considered for certification were completely new territory for us and presented us with some major challenges,” explains Sebastian Heidrich.
Jan Schikora, Regulatory Affairs Expert at Harro Höfliger’s Device Services, is very well acquainted with the regulatory pitfalls and knows how to overcome them: “We not only advise on the development or optimization of devices, but also on all matters relating to approval. Conformité Européenne marking is required for the European market to confirm that the product functions properly and meets all relevant safety requirements.”
To obtain the CE mark, manufacturers must undergo a comprehensive conformity assessment procedure and identify the applicable legal framework themselves. Jan Schikora: “We offer support for the entire process, from advising on the correct procedure to preparing the required technical documentation, which includes classification, product description, information on performance, safety, data protection, and numerous other details. Assigning a medical or diagnostic product to one of the various risk classes correctly right from the start is important and saves time.”
Sebastian Heidrich confirms this. “We benefited from Jan Schikora’s extensive regulatory expertise. His well-structured, results-oriented approach contributed to a smooth process and the rapid approval of our smart device in the fall of 2025. Ultimately, we were able to save resources and valuable time, which we invested in providing people with even more precise technologies for their healthcare.”